Full Time – Anovus Certified
PROFESSIONAL CERTIFICATE IN PHARMACOVIGILANCE (PCPV)
DURATION: 3 MONTHS, WEEKEND COURSE
PHARMACOVIGILANCE
‘ Pharmacovigilance is the science and activities relating to the Detection, Assessment, Understanding and Prevention of adverse drug reactions or any other possible drug related problems ’. WHO 2002
Importance of Post Marketing Surveillance
Known pharmacology of the drug can’t predict bizarre reaction.
- 30,000 people need to be treated to detect drug reaction having incidence of 1 in 10,000.
Pharmacovigilance Programme of India (PVPI)
Pharmacovigilance programme of India (PVPI) was launched in July 2010.
Goal
To ensure that the benefits of use of medicine outweighs the risks and thus safeguard the health of the Indian population.
CAREER PATH IN PHARMACOVIGILANCE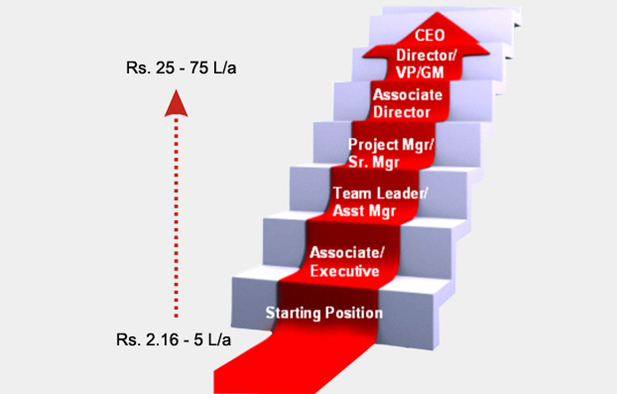
GLOBAL OPPORTUNITIES
As per Frost & Sullivan, the world pharmacovigilance market was worth $1859.9 million in 2008 and is estimated to reach $2252.2 million in 2015.
PROGRAM MODULES
- Clinical Research
- Pharmacology
- Pharmacovigilance
- ADR forms
- Risk Management
- Signal Detection
- Data Mining
ANOVUS ADVANTAGE
- Get professionally qualified from North India’s leading institute
- Job-oriented Specialized training in Pharmacovigilance
- Course designed by industry professionals as per the latest requirements of the industry
- Latest industry software will be taught
- Experienced faculty from the industry
- Case studies based teaching
- Excellent career progression
STUDENTS’ PLACEMENTS
Large numbers of our students have been placed at Quantum Solutions, Chandigarh over the last several years; one of India’s leading Pharmacovigilance organizations.
IT majors including TCS, Cognizant, Wipro, Accenture, Genpact, HCL are among a host of others who have dedicated a business vertical entirely for pharmacovigilance.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS
- BAMS
- BDS
- BHMS
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- B.Sc (Nursing)
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
PROFESSIONAL CERTIFICATE IN CLINICAL DATA MANAGEMENT(PCCDM)
Duration: 3 MONTHS, WEEKEND COURSE
CLINICAL DATA MANAGEMENT
Clinical Data management is a discipline that describes the processing of the data that is generated during a clinical trial.
DEMAND IN THE INDUSTRY
The Clinical Research industry in India is expected to touch Rs. 7,000 crores and will employ additional 50,000 people by 2014. There is immense potential in this field as there are more than 200 large and small Pharmaceutical and Contract Research Organizations conducting clinical research and clinical data management in India. Thus, there is a rising demand for clinical research professionals, making it an interesting career option with massive growth potential. ANOVUS provides the best and most comprehensive training programs to bridge this gap.
ANOVUS ADVANTAGE
Case Report Form (CRF) designing, CRF annotation, database designing, data-entry, data validation, discrepancy management, medical coding, data extraction, and database locking are assessed for quality at regular intervals during a trial.
- Hands-on training on the latest software for CDM will be provided.
STUDENTS’ PLACEMENTS
Our students are already working in Clinical Data Management organizations in Chandigarh and at several other places in India.
JOB OPPORTUNITIES
IT MAJORS: Accenture, Wipro, Genzyme, Genpact, HCL, TCS and Reliance.
PHARMA COMPANIES: Bayer, PGI Chandigarh,Bristol Meyers Squibb, Cadila Healthcare Ltd,
inVentive Health, iGATE, Intas Pharma, IPCA, IRL Research Ltd, Novartis, NovoNordisk, , SRL Ranbaxy, Roche, Sanofi Aventis, , Excel Life Sciences, Glaxo Smithkline, Glenmark, GVK, Hindustan Lever Limited, WNS Global, , Wockhardt, Johnson & Johnson, Jubilant Clinsys, Kendle, Lambda, Merck, Medpace International, Metropolis , Max Neeman etc.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS
- BAMS
- BDS
- BHMS
- B.Sc (Nursing)
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
Final year students & Interns from these courses may also apply.
PROFESSIONAL CERTIFICATE IN CLINICAL SAS (PCCSAS)
Duration: 3 MONTHS, WEEKEND COURSE
SAS (Statistical Analysis System), the world’s fastest and most powerful software for data management, data mining, report writing, statistical analysis, business modeling, applications development and data warehousing. SAS can provide you excellent job opportunity in India as well as abroad with a hefty package.
Total No. of SAS Certified Professionals all over the world is 50,000 only (www.sas.com).
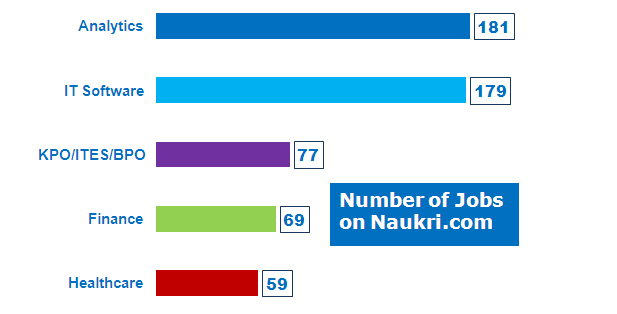
SAS Jobs by Job Function In terms of number of job opportunities by functional area, Analytics topped the list with 181 jobs while IT Software being second in the list with 179 jobs ( Naukri .com – 17 th August 2013).
Starting package of a SAS Programmer in India (according to payscale.com)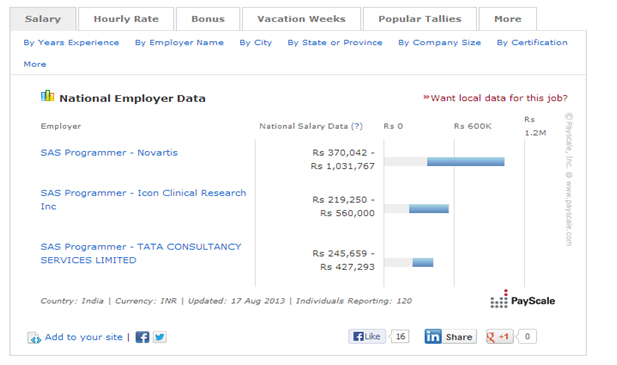
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- Graduates/ Post Graduates (Medical/Non-Medical)
- B.Sc./M.Sc.
- B.Sc./M.Sc.(IT)
- B.Sc./M.Sc. (MATHS)
- B.Sc./M.Sc. (PHYSICS)
- B.Sc./M.Sc. (CHEMISTRY)
- BE/B.Tech
- MBBS
- BAMS
- BDS
- BHMS
- B.Sc (Nursing)
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm /M.Pharm
- B.Com
- BBA
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics, Biotechnology etc.
Final year students & Interns from these courses may also apply.
ACCELERATED DIPLOMA IN CLINICAL RESEARCH + SPECIALISATION (CDM, ADVANCED SAS, PHARMACOVIGILANCE)
DURATION – 3 MONTHS, WEEKEND COURSE
This first-of-its-kind programme in North India is comprehensively-structured to match academic standards with industry requirements. The mix of Clinical Research, Clinical Data Management, Advanced SAS and Pharmacovigilance curriculum along with Management Module and Soft Skills in the course ensures an advantage to move up the corporate ladder faster.
The course with its comprehensive curriculum makes a world of opportunities available to students when they step into the industry. The course ensures that students get a quick entry into the industry for faster career success.
Students also would receive hands-on training in the latest software as applicable to their choice of subject in order to get an extra advantage over their counterparts.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS
- BAMS
- BDS
- BHMS
- B.Sc (Nursing)
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
Final year students/Interns from these courses may also apply.
INTERGRATED DIPLOMA IN CDM, CLINICAL SAS & PHARMACOVIGILANCE
DURATION – 6 MONTHS, WEEKEND COURSE
This unique course has been structured to match the industry requirements and academic standards. The mix of Clinical Data Management, Clinical SAS and Pharmacovigilance curriculum along with Management Module and Soft Skills has been especially designed to teach both theory and practical in order to ensure that the student gets an extra edge over others & are able to fulfill the demands of today’s corporate sector.
The course with its comprehensive curriculum develops the opportunity available to students when they step into the industry. The course ensures that students get a quick entry into the industry for faster career success.
Students also would receive hands-on training in the latest software in order to get an additional benefit over their competitors.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS
- BAMS
- BDS
- BHMS
- B.Sc (Nursing)
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
Final year students/ Interns of these courses may also apply.
INTERGRATED DIPLOMA IN CLINICAL RESEARCH, CDM, CLINICAL SAS & PHARMACOVIGILANCE
DURATION – 6 MONTHS, WEEKEND COURSE
This distinctive course has been especially-designed to counterpart with the latest demand in Clinical Research Industry and educational standards. The combination of Clinical Research, Clinical Data Management, Clinical SAS and Pharmacovigilance curriculum along with Management Module and Soft Skills has been especially designed to teach both theory and practical in order to ensure that the student gets an extra edge over the others & is able to fulfill the demands of today’s corporate sector.
The course with its all-inclusive curriculum develops the opportunity available to students when they step into the industry. The course ensures that students get a quick entry into the industry for faster career success.
Students also would receive the training in latest software in each subject in order to get an additional benefit over the others in the industry.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS, MD
- BAMS
- BDS
- BHMS
- B.Sc (Nursing)
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
Final year students/ Interns of these courses may also apply.
INTEGRATED DIPLOMA IN CLINICAL RESEARCH + SPECIALIZATION (CDM, ADVANCED SAS, PHARMACOVIGILANCE)
DURATION – 6 MONTHS, WEEKEND COURSE
This programme is comprehensively-structured to match academic standards with industry requirements. The mix of Clinical Research, Clinical Data Management, Advanced SAS and Pharmacovigilance curriculum along with Management Module and Soft Skills in the course ensures an advantage to move up the corporate ladder faster.
The course with its comprehensive curriculum develops the opportunity available to students when they step into the industry. The course ensures that students get a quick entry into the industry for faster career success.
Students also would receive training in the latest software which would be applicable to their choice of subject in order to get an extra advantage over their counterparts in the industry.
ELIGIBILITY
Minimum eligibility criteria for application to the course would be either of the following:
- MBBS, MD
- BAMS
- BDS
- BHMS
- Physiotherapy and Occupational Therapy Graduates
- B.Pharm/M.Pharm
- Graduates/ Post Graduates in Sciences/Life sciences/ Bio Sciences with any of the following subjects-Chemistry, Botany, Zoology, Biochemistry, Bioinformatics, Microbiology, Genetics and Biotechnology.
Final year students/ Interns of these courses may also apply.

